Previous clinical trials run at Novatrials
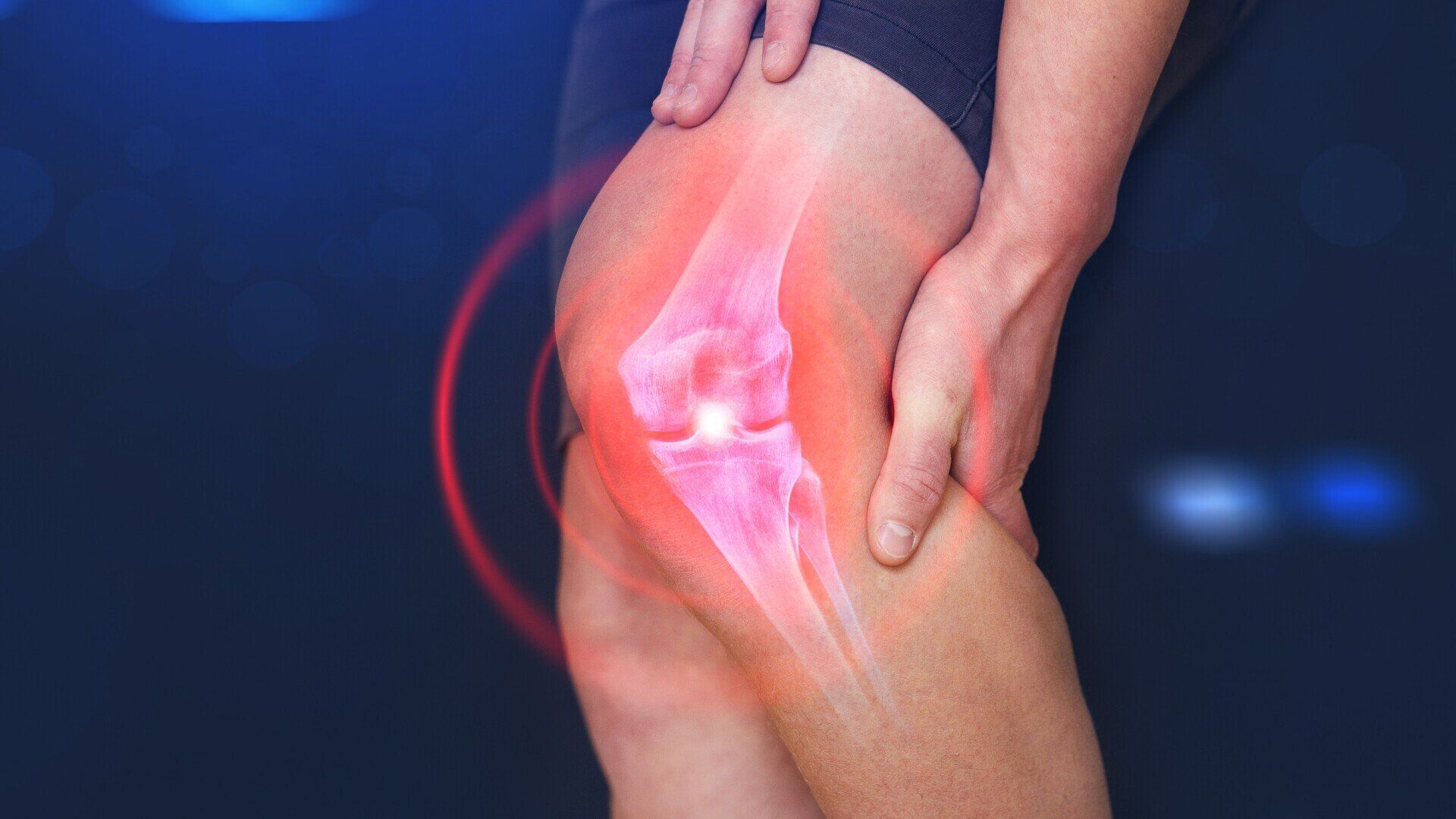
Osteoarthritis of the Knee
Chronic knee pain holding you back?
If you suffer from chronic pain or aching in your knee, you may have osteoarthritis (OA) - a painful joint condition that can interfere with all aspects of daily life. If you are living with chronic knee pain and have not received lasting relief from medications, you may qualify for a new clinical research study. The study is for an investigational medication for chronic knee pain.
If you qualify and choose to join the study, you will receive study-related care at no cost and will also be reimbursed for your travel to attend study visits.
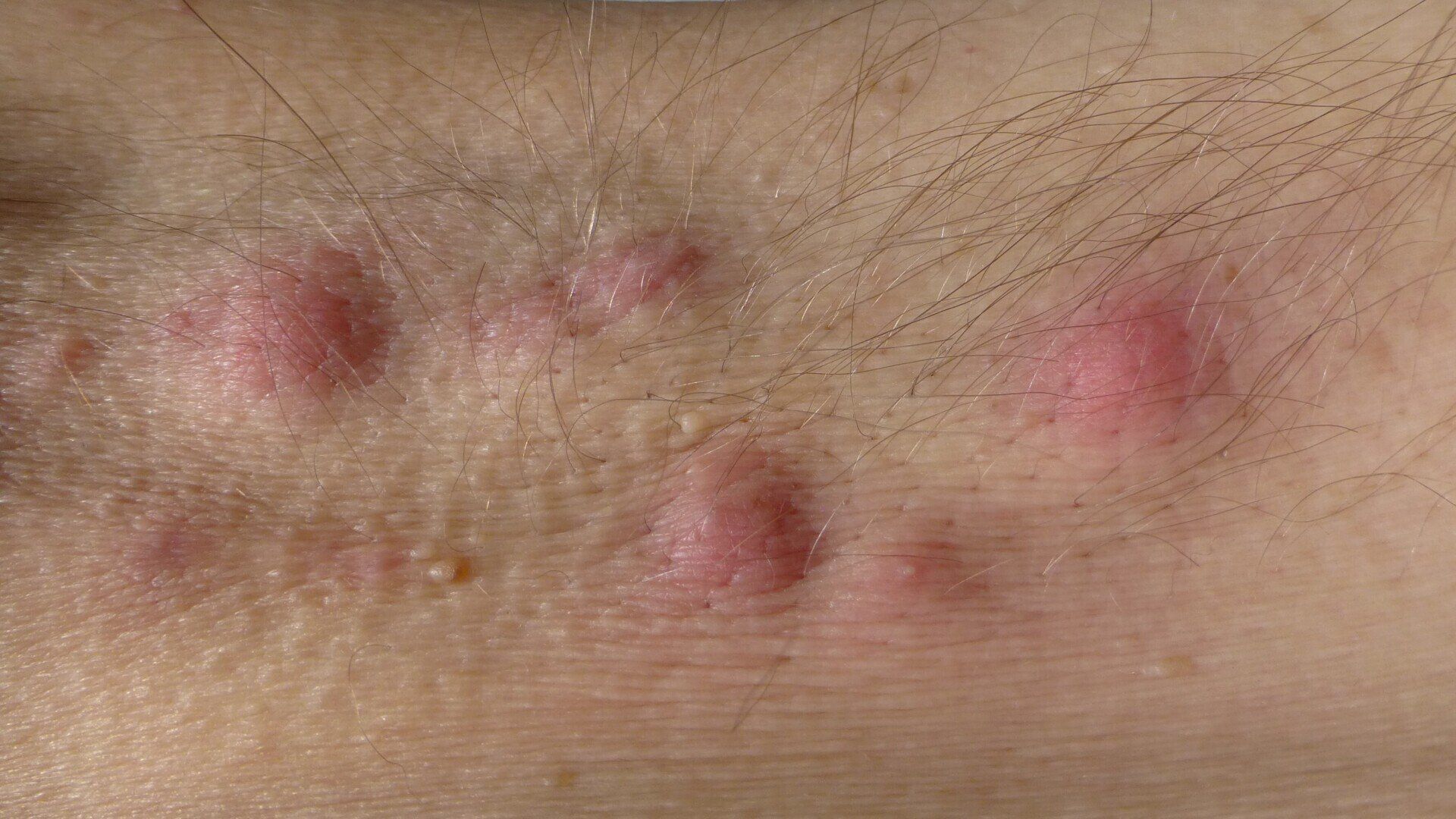
Hidradenitis Suppurativa
Hidradenitis Suppurativa (HS) is a chronic inflammatory skin disorder causing painful lumps/nodules to form in areas where the skin rubs together such as armpits and groin. Novatrials is now running a trial of a potential new medication for HS.
If you are suffering with HS you may be eligible to join the trail if you:
• are aged 18 and over;
• diagnosed with or have HS signs/symptoms;
• can attend study vists at Kotara
Participants receive study treatment from our dedicated medical team at no cost. Participants are also reimbursed for travel expenses for study visits.
Omicron Covid-19 Vaccine
Help us find a vaccine for Omicron We are seeking volunteers to participate in a Phase 3 clinical trial of a potential Novavax Covid-19 vaccine booster aimed at the Omicron variant. If you are aged over 18 years and have already received Covid-19 vaccinations of the following:
*At least 3 doses of an mRNA Vaccine (Either Pfizer and/or Moderna) at least 3 months ago
We want to hear from you if you meet any of the above and can attend 6 appointments over 8 months at Novatrials clinic in Kotara. Participants receive a vaccination and study-related health care from a dedicated medical team at no cost. Participants also receive $90 per visit for travel expenses for attending appointments.
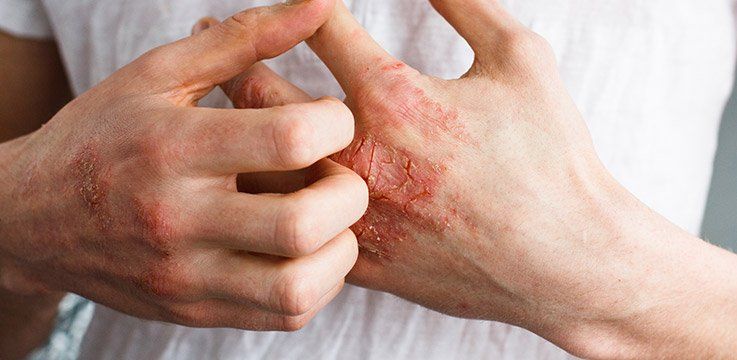
Atopic Dermatitis
Suffering with Eczema (Atopic Dermatitis). You may be eligible to participate if you:
* are aged 18-65 years of age (inclusive)
* have been diagnosed with moderate to severe Eczema (Atopic Dermatitis)
* have not had adequate response to other Eczema treatments in the last 12 months.
Participants receive study medication and study-related care from our dedicated medical team at no cost. Participants also receive travel expenses for attending appointments.
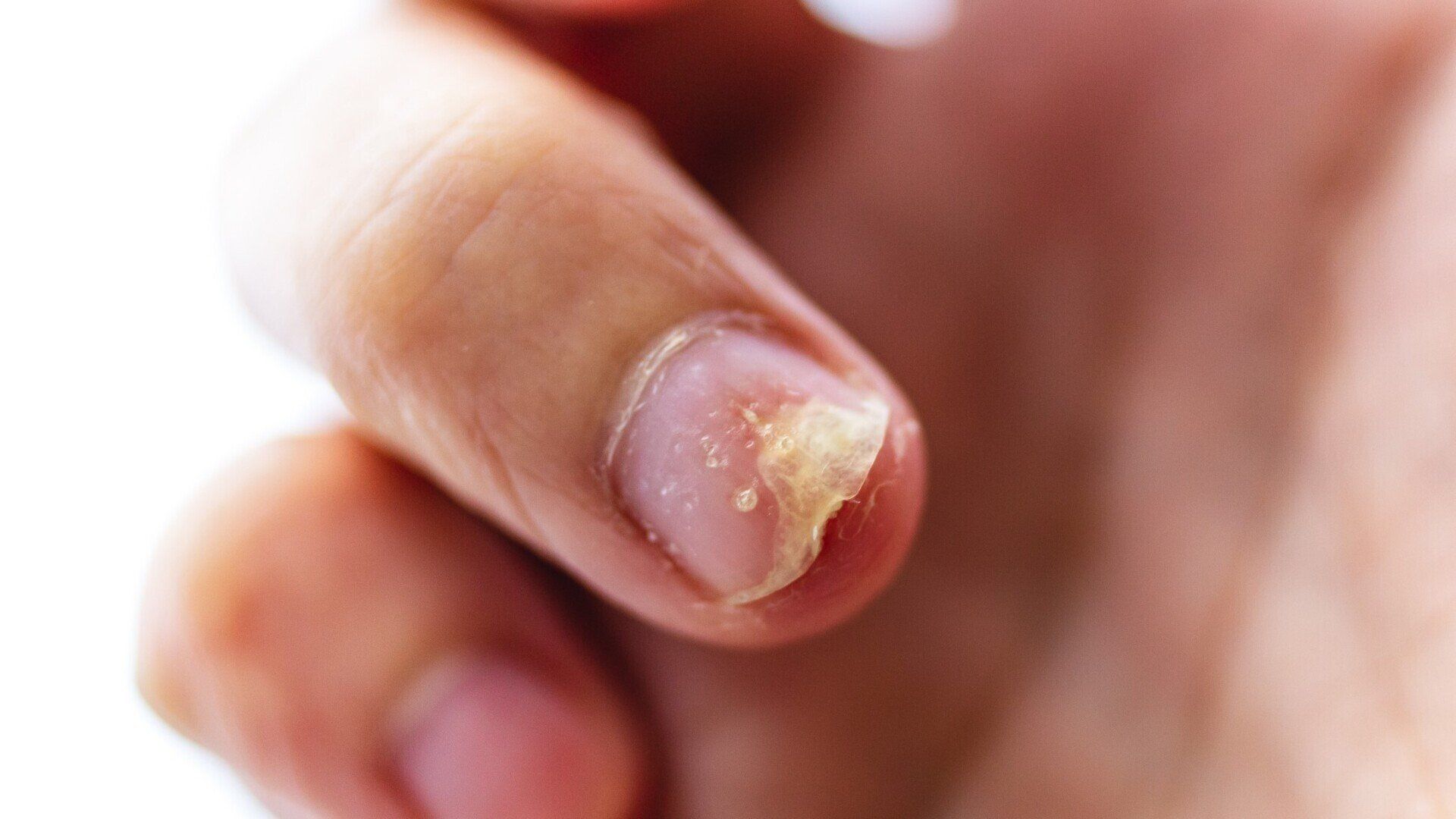
Nail Psoriasis
Do you have nail psoriasis? If so, you may be interested in a medical research study of a new topical medication.Individuals who qualify must be:
• 18 – 80 years old
• Diagnosed with fingernail psoriasis for at least 3 months
• Diagnosed with or have a history of plaque psoriasis or psoriatic arthritis
The study is 32 weeks long with a 2 week follow-up. For 16 weeks, patients will treat one side with active medication and one side with placebo. For another 16 weeks, all patients will receive active medication to treat both sides, with the option to treat whole body. The study is being conducted at multiple clinical sites across Australia.
Alopecia
Do you have Alopecia? Novatrials are seeking volunteers with Alopecia to join a clinical trial of a potential new treatment. You may be eligible for the trial if:
* You are aged between 18-65 years
* Have at least 25% or greater hair loss
* You can attend appointments at our Kotara clinic (8 visits over 8 months)
Participants receive study medication and study-related care by a dedicated medical team at no cost. Participants also receive travel expenses for each clinic appointment.
Kidney Disease caused by Type 2 Diabetes
Having high levels of a protein called albumin
in your urine could be a a sign of kidney damage. We are running a trial of a potential new medication that may help improve your kidneys. (The same medication is also being trialed for blood glucose control with associated weight loss).
You may be eligible to join the trial if you:
* Are aged between 18-75 years of age (inclusive)
* Have Type 2 Diabetes
* Have Diabetic Kidney Disease
(if you don't know if you have high albumin, contact us to have a simple blood test to check for Albuminuria)
The trial involves 6 visits to the Kotara clinic over 4 months.
Participants receive study medication and study-related care from our dedicated medical team at no cost. Participants also receive travel expenses.
Chronic Plaque Psoriasis
This trial is looking at a new skin gel treatment option for mild to moderate Chronic Plaque Psoriasis. To participate the skin gel is applied to the psoriasis plaque for 28 days. There are 7 appointments at the clinic over 8 weeks. Study medication and study-related care from a dedicated medical team provided at no cost.
Heart Failure
This trial is looking at a potential new treatment option for heart failure. You may be eligible to participate if you have been diagnosed with chronic heart failure and have not had a serious heart event (such as a heart attack, stroke, or major heart surgery) within the last 90 days.
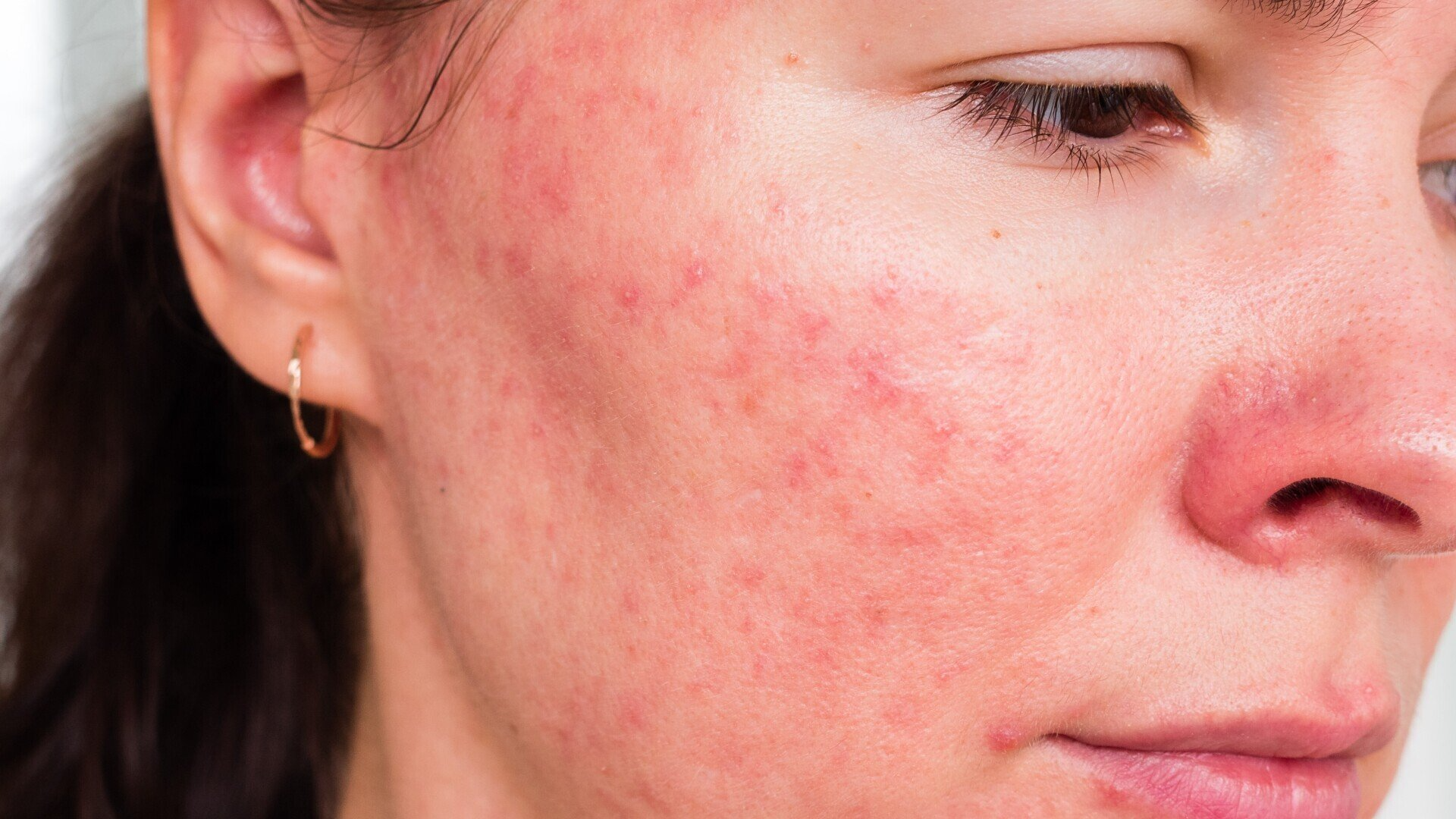
Rosacea (Papulopustular)
Do you regularly have flushing or redness with an acne-like breakout on your forehead, cheeks, nose and surrounding area or chin?
Have you been diagnosed with Rosacea?
A research study is now underway for an investigational new treatment for Rosacea and you could be eligibil for this 8 week study.
Medication and check-ups are included and travel costs will be reimbursed.